Abstract
Background: DLBCL is the most common type of non-Hodgkin lymphoma, and 30-40% of patients develop R/R DLBCL (Sehn LH and Salles G. N Engl J Med 2021), which is associated with substantial treatment cost. Previous studies evaluated the total healthcare costs by line of therapy (LoT) (Danese MD, et al. Leuk Lymphoma 2017; Tkacz J, et al. Leuk Lymphoma 2020; Yang X, et al. Oncologist 2021; Mutebi A, et al. ISPOR 2022); however, these studies have not been updated with more recent real-world data. This analysis evaluated the total cost of care and health resource utilization associated with R/R DLBCL therapies by LoT, including those recently approved, to understand the impact of novel therapies such as chimeric antigen receptor T-cell (CAR-T) therapy on total cost of care and resource use in the R/R DLBCL setting.
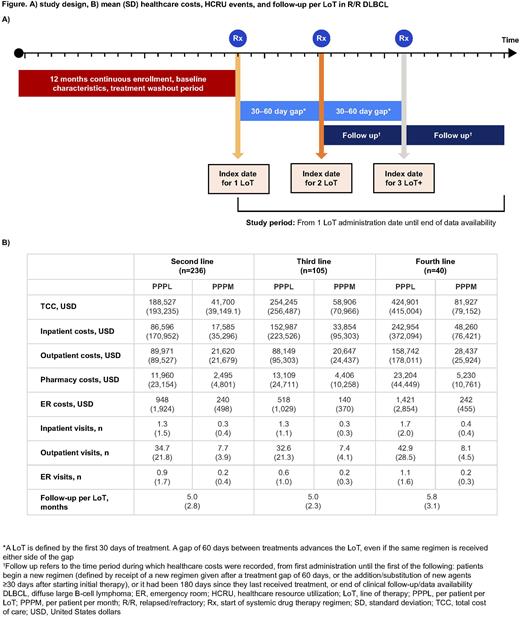
Methods: We evaluated healthcare costs and resources for each regimen in the second-line (2L) setting and beyond in patients who received rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or R-CHOP-like therapy (at least rituximab, cyclophosphamide, and one corticosteroid) as first-line (1L) treatment for DLBCL between January 1, 2015 and June 30, 2021 using the IQVIA PharMetrics® Plus Database (Figure A). Costs were assessed for each LoT from the first administration date until the beginning of a new regimen (defined by receipt of a new regimen given after a treatment gap of 60 days, or the addition/substitution of new agents ≥30 days after starting initial therapy [adapted from Yang X, et al. Oncologist 2021]), a treatment gap of 180 days, or end of follow-up. Systemic therapies initiated <30 days before the start of CAR-T therapy or stem cell transplant (SCT) were considered pre-treatment and were included in the costs for only that LoT, to be inclusive of conditioning and bridging treatments. Total cost of care (TCC) was defined as all-cause healthcare costs. Healthcare resource utilization (HCRU) costs and events were defined using inpatient, outpatient, pharmacy, and emergency room (ER) categories. Baseline characteristics, including age and payer type at index, sex, and US region were assessed in the 12 months pre-index. TCC was reported as per patient per month (PPPM) and per patient per LoT. Data were descriptively assessed.
Results: The final analysis cohort included 236 patients with a median age at first ≥2L treatment of 60 years (interquartile range [IQR] 54-64), 37% of patients were female, and median follow-up from 1L initiation was 21 months [IQR 13-35]. Of the patients who received 2L treatment, 44.5% (105/236) received third-line (3L) treatment and 16.9% (40/236) received fourth-line (4L) treatment. CAR-T therapy use increased in later LoTs (5.9% in 2L, 5.7% in 3L, and 17.5% in 4L). SCT was frequently used across all LoTs (25.8% in 2L, 50.5% in 3L, and 32.5% in 4L).
The mean TCC PPPM by LoT was $41,700 (standard deviation [SD] $39,767) for 2L, $58,906 (SD $70,966) for 3L, and $81,927 (SD $79,152) for 4L. Mean PPPM HCRU costs generally increased by LoT for inpatient and pharmacy claims, and costs were highest in 4L for all HCRU categories (Figure B). PPPM HCRU events were highest in the 4L setting for outpatient visits only (Figure B).
Conclusion: This economic analysis demonstrated that total healthcare costs in R/R DLBCL increase with each additional LoT. Cost estimates by LoT appeared higher than previously reported (Tkacz J, et al. Leuk Lymphoma 2020; Mutebi A, et al. ISPOR 2022), and may be a result of the increase in SCT and CAR-T therapy use. Inpatient costs represented the highest percentage of total costs, followed by outpatient costs. New 1L treatments that are both efficacious and cost-effective in DLBCL are needed to reduce the economic burden to the healthcare system in the R/R DLBCL setting.
Disclosures
Gatwood:Kite: Speakers Bureau; Sanofi: Speakers Bureau; Jazz Pharmaceuticals: Speakers Bureau; GlaxoSmithKline: Research Funding; Genentech: Consultancy; AstraZeneca: Research Funding; Janssen Pharmaceuticals: Consultancy; Merck & Co.: Consultancy, Research Funding. Masaquel:Genentech, Inc.: Current Employment; Roche: Current equity holder in private company, Current holder of stock options in a privately-held company. Ross:Genesis Research: Current Employment, Current equity holder in private company. Sheinson:Genentech/Roche: Current Employment; Roche: Current equity holder in private company, Current holder of stock options in a privately-held company. Hossain:Genentech: Current Employment; Roche: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months; Legend Biotech: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Li:Roche: Current Employment. James:Genentech: Current Employment, Current equity holder in publicly-traded company; Sanofi: Current equity holder in publicly-traded company. Fox:Genentech, Inc.: Current Employment, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal